Published student research suggests that Streptococcus salivarius M18, a bacteria producing antimicrobial and pH-altering enzymes, may be used to suppress cavity formation.
On October 16, 2023, research from Western Reserve Academy’s afternoon BioBuilder program was published online through BioTreks, a peer-reviewed high school synthetic biology journal.
The BioBuilder team, comprised of 20 students meeting three times per week, explored initial research performed by Loretta Wang ’25, who examined the effects of two Streptococcus bacteria in the mouth. Then, the winter team collected key background information from academic sources to design a paper. Using this research, the spring team drafted and submitted a paper for publication, theorizing the use of a bacterial paste to prevent the development of dental caries, more commonly known as cavities.
Cavities are primarily caused by plaque formation. Bacteria that colonize plaque, such as Streptococcus mutans, produce acids that damage enamel and weaken teeth. S. mutans is a spherical (coccus), facultative anaerobic bacteria. Facultative anaerobes grow best when oxygen is present in the environment, but are capable of producing energy through fermentation in oxygen-free conditions. Therefore, S. mutans is able to grow both on a tooth’s surface and beneath a layer of plaque.
Streptococcus salivarius, another bacteria colonizing the mouth, is a harmless bacteria present in humans immediately after birth. S. salivarius M18 produces two key enzymes combating the damage from S. mutans: dextranase and urease. Dextranase breaks down dextran in the plaque of decaying teeth, thereby reducing plaque formation. Urease uses the compound urea, which is also found in urine, to generate ammonia. The production of this ammonia neutralizes the damaging acids of S. mutans.
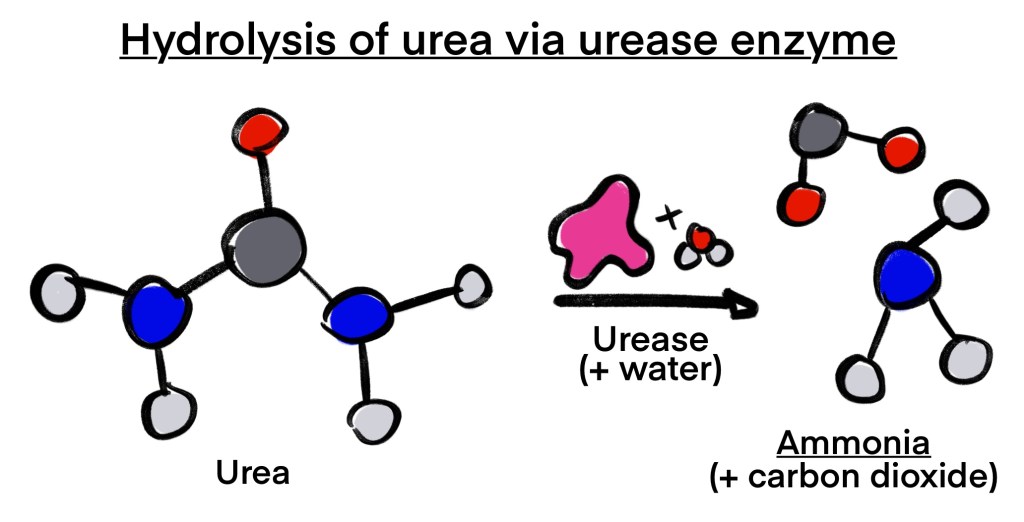
The BioBuilder team at WRA sought to use the beneficial properties of S. salivarius as a form of cavity prevention. To avoid the costly, painful treatments for patients with cavities, the team designed a paste which inserts the dextranase and urease genes from S. salivarius into Rothia aeria.
Three genes of urease (ureA, ureB, and ureC) and one dex gene were introduced into a pJPC13 plasmid. A plasmid is a circular DNA molecule in bacteria. In synthetic biology, scientists use plasmids to transfer genes from one host organism to another.
The pJPC13 plasmid includes a lac promoter, lac terminator, and chloramphenicol resistance marker. The promoter tells RNA polymerase where to start cutting the desired genes, while the terminator indicates the end of the target segment. The cholramphenicol marker is used to isolate successfully transformed bacteria on a chloramphenicol agar plate. Agar is a gelationous medium for growing bacteria that provides the nutrients needed for certain bacterial species to grow. R. aeria is not naturally resistant to chloramphenicol, an antibiotic. Therefore, if a colony of R. aeria is not successfully transformed with the pJPC13 plasmid, it will not have the resistance from the plasmid and will die on the chloramphenicol plate, leaving only colonies that have been transformed.

This transformation process occurs through electroporation, which places bacterial cells (in this case, R. aeria) and plasmids in an electroporator. The electroporator then applies short electric pulses; these pulses create small pores that allow the plasmid to slip into the cells. The cells naturally close up, trapping the desired plasmid inside.
The team selected R. aeria A 1-17B as the bacterial chassis because it is an early colonizer of human teeth, like S. mutans and S. salivarius. Therefore, since the project is not introducing foreign bacteria into the mouth, it would likely have a minimal impact on natural microbiome of the mouth. The project design envisioned the utilization of R. aeria through a dental paste. The paste was designed for application after dental cleaning, promoting a healthy microbiome in the mouth that slows the formation of cavities in the months after application.
Though this project remains theoretical, safety must be considered. Consultations with professionals would confirm the safe use of R. aeria, a Biosafety Level 1 organism that presents minimal risk to humans. Dextranase is another safe enzyme to work with, as dextran is naturally found in beets and sugarcane. However, the use of urease becomes a concern. Urease has been shown to have toxic effects on human cells, but special alterations to growth medium can limit urease levels. Should this project continue in future years of WRA’s Synthetic Biology courses, safety would be an area where further research and experimentation is required.
Ideally, the WRA BioBuilder team hopes that their research can contribute to a better form of dental treatment for all, preventing cavities for years to come.
Sources:
- Amos, L., Danzhen, L., Dhingra, P., Frohring, E., Haslinger Johnson, I.,
Kovacevic, S., Li, D., Liu, C., Ma, Mathur, A., Muligande, S., Nguyen, A.,
Qin, R., Reed, C., Shimpi, A., Wang, L., Xue, C., Yang, S., Zamarro, M., &
Zhou, Y. (2023). Designing a preventative treatment for Streptococcus
mutans induced dental caries using the antimicrobial properties of
Streptococcus salivarius M18. BioTreks, 8, 9-15. https://biotreks.org/
e202311/


Leave a comment